Current status
Research groups with experience in material characterization of biological tissue were invited to join this initiative. We are currently in test round 2 of the first test campaign on:
Uniaxial tensile testing of soft biological tissues
Contact us if you want to be involved in future test campaigns.
News
After having received applications from all over the world, the selected participants for the first test campaign on uniaxial tensile testing of porcine aorta are shown below.
Background
Context
Computational modeling is successfully used to address safety-critical issues and to provide evidence in the regulatory approval process in the aerospace, automotive, and nuclear industries for quite some time. The healthcare industry is also moving towards this in silico approach, which can be used in all phases of R&D, from the design of the product, over development to the regulatory approval and post-marketing stage. Besides a clear definition of the context of use and a rigorous verification and validation process (as provided by ASME V&V40), the quality of the model input has a great influence on the model credibility. Therefore, the accurate representation of the involved biological tissues is identified as a significant barrier for in silico medicine to truly impact the healthcare industry and become an accepted method for evidence generation in the regulatory approval process.
Problem statement
Although the scientific literature abounds with articles experimentally characterizing biological tissues, the reported parameters often show a high variability, partially due to the absence of widely recognized testing standards for biological tissue.
Aim
The aim of the challenge is to achieve community consensus regarding the testing protocols for material characterization of biological tissue and disseminate this consensus to the relevant standards bodies (i.a. ISO & ASME). The physical properties of interest can be thermal, mechanical or electrophysiological. In the first stage, this challenge will focus on mechanical tissue properties collected through in vitro experiments.
In vitro mechanical characterization of biological material entails many aspects, including tissue collection, preservation, preparation, experimental set-up, protocol development, execution and post processing. A wide variety of testing methods exist which are often specific to the tissue, the material parameters of interest and the clinical situation of interest. Standardization in this broad field is non-trivial. The challenge will therefore be organized in different test campaigns, each time focusing on a single aspect.
Test campaigns
Four phases
For each test campaign the following four phases (click for more information) are identified:
-
Variability quantification (test round 1)
Quantify the variability of material mechanical properties resulting from 'identical' experiments performed by different research groups. The participants are requested to apply their own methodology to perform the experiments on a sample set provided by C4Bio, and send back a description of their methodology along with the obtained results. During a colloquium to be attended by all participants, C4Bio will bundle all received data and report on the variation in the applied methodology as well as the variability of the test results.
-
Consensus definition (colloquium)
During the same colloquium, all participants will engage in a discussion to reach a consensus methodology covering each aspect of the test, with the aim of maximizing practicality, accuracy, reproducibility and acceptability. Apart from the participating teams, experts from standards-related organizations will join the discussion.
-
Consensus evaluation (test round 2)
Evaluate the benefit of the consensus methodology. Participants will then repeat the experiment based on the consensus methodology during a second testing campaign. The reduction in interlaboratory variability as compared to the first testing round will be quantified. The outcome will again be discussed among all participants for small improvements on the first version of the consensus methodology as well as on guidelines for reporting.
-
Dissemination
Make the outcome publicly available. The final consensus methodology will be published as guidelines for experimentalists through a white paper, which the participants will be invited to co-author. Secondly, the data collected in the second round of experiments will be made publicly available in a centralized database.
This procedure will be performed for common testing methods (uniaxial tensile testing, planar biaxial tensile testing, extension-inflation testing, unconfined and confined compression testing, etc.) and for relevant tissue types, each time reaching a new consensus methodology as well as expanding the database.
Current test campaign
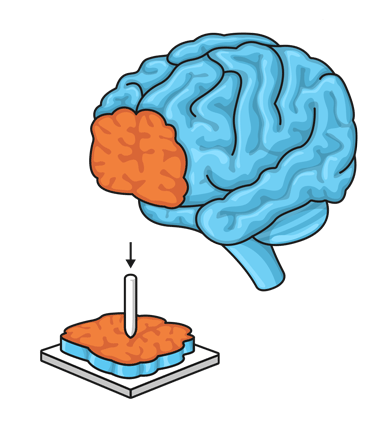
The first test campaign has started in January 2021 and is focusing on uniaxial tensile testing of soft biological tissue (in casu porcine aorta) to obtain linearized stiffness moduli and ultimate stress and strain values.
The tensile test is a fundamental test in engineering, largely documented and already standardized for metals, composites and plastics. This kind of loading and its outcome parameters can be considered as one of the most common, simple and useful in the biomechanical engineering field, making it the ideal place to start.
Currently, the campaign is in the tensile test is iterating between the consensus definition and consensus evaluation. A list of contributing authors can be found here .
Download details on the current test campaign. (PDF)Upcoming test campaigns
The second test campaign will tackle planar biaxial tensile testing, which is another very commonly used method for tissue characterization that has various sources of variability. The tentative registration deadline of this campaign will be April 1st 2022.
Suggestions?
Do you have suggestions for an upcoming test campaign? Contact us
Who can join?
We invite all research groups with experience in material characterization of biological tissue to join this initiative. The prerequisites are:
- The availability of infrastructure suitable for current test campaign.
- The clearance to test biological tissue in your lab.
- A demonstrated experience in mechanical experiments on biological tissue through scientific references.
- The willingness to publicly share the experimental results (i.e. in the resulting white paper and database).
For practical reasons, the number of participating groups is limited to 20. A selection will be made based on:
- Expertise and track record on testing of biological tissue.
- Potential for the group to join in the following campaigns (i.e. expertise in and facilities for more than one test method).
- Geographical spread.
Why join?
Participants taking part in a test campaign will:
- be part of a unique consortium consisting of academic and industry experts at the forefront of standardization in material characterization of biological tissue.
- drive forward the field of in silico medicine by contributing to a key breakthrough in one of the most significant barriers to ubiquitous adoption of computational biological models.
- be involved as co-authors in the resulting white paper.
Timeline
Uniaxial Tensile Testing
- 16/11/2020 - Call for participants opens
- 04/01/2021 - Call closes
- 18/01/2021 - Notification of acceptance
- 01/02/2021 - Samples are sent for test round 1
- 01/04/2021 - Deadline for submission results test round 1
- 01/06/2021 - Colloquium 1 with discussion on results & methodology (online)
- 29/06/2021 - Colloquium 2 with discussion on results & methodology (online)
- 04/10/2021 - Samples are sent for test round 2
- 05/11/2021 - Deadline for submission results test round 2
- 30/06/2022 - First draft of white paper is distributed to the co-authors
Team
C4Bio was conceived by the Research & Technology Working Group, driven by the VPHi (the international scientific society for in silico medicine) in the context of the Avicenna Alliance (the academia-industry alliance for precision medicine). Practical organization is done by FIBEr (KU Leuven Core Facility for Biomechanical Experimentation). The core members, partners and organizers of the initiative are:

Karine Bruyere-Garnier
LBMC, Univ Lyon, Univ Gustave Eiffel Core member
Alex Caulk
Medtronic Core member
Martina Contin
VPH institute Core member
Kimberly Crevits
FIBEr, KU Leuven Organizer
Nele Famaey
FIBEr, KU Leuven Core member, Organizer
Heleen Fehervary
FIBEr, KU Leuven Core member, Organizer
Liesbet Geris
VPH institute, KU Leuven and University of Liège Core member
Yoann Lafon
LBMC, Univ Lyon, Univ Gustave Eiffel Core member
Leartiker
Partner
Thierry Marchal
Avicenna Alliance, Ansys Core member
Afshari Payman
Johnson & Johnson Core member
Venkateswaran Perumal
Stryker Core member
Johan Philips
FIBEr, KU Leuven Partner, Organizer
Markus Reiterer
Medtronic Core member
Randy Schiestl
Boston Scientific Core member
Martin Tanaka
Western Carolina University, ASME Partner
Adriana Wilmots
FIBEr, KU Leuven OrganizerWe are still looking for experts regarding the characterization of thermal or electrical properties of biological tissue. Do you have experience? Contact us